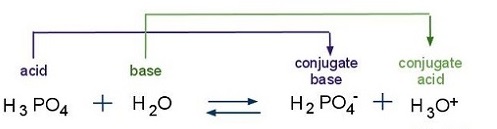
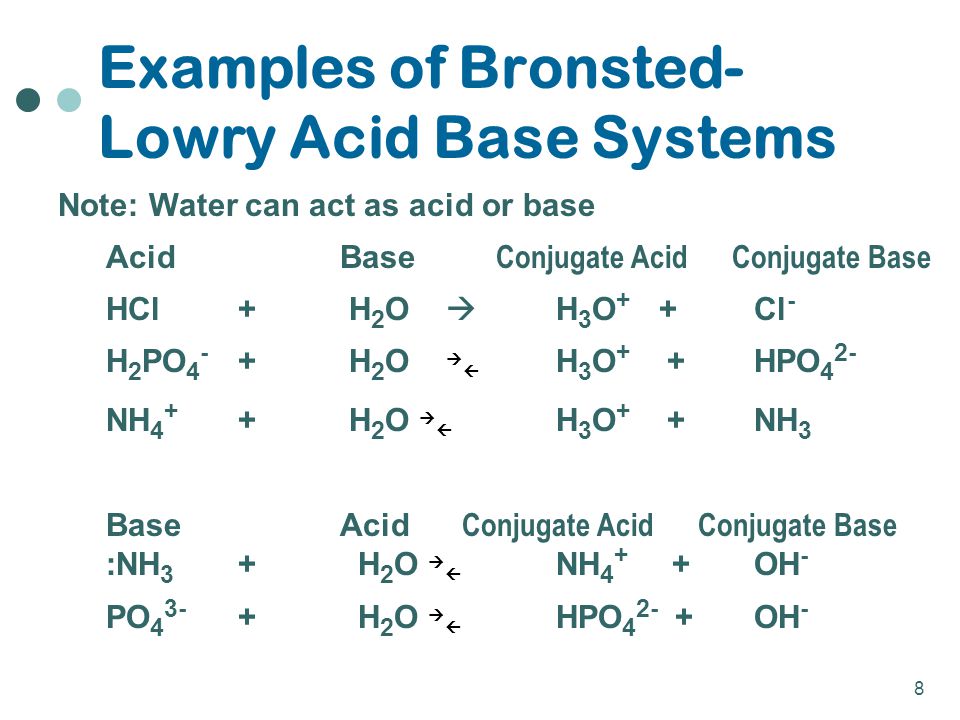
SOLVED: which of the following is not a conjugate acid base pair?A) H3PO4 and HPO4^2-B) H2PO4^- and HPO4^2- C) HPO4^2- and PO4^3-D) H3PO4 and H2PO4^-E) H2O and H3O

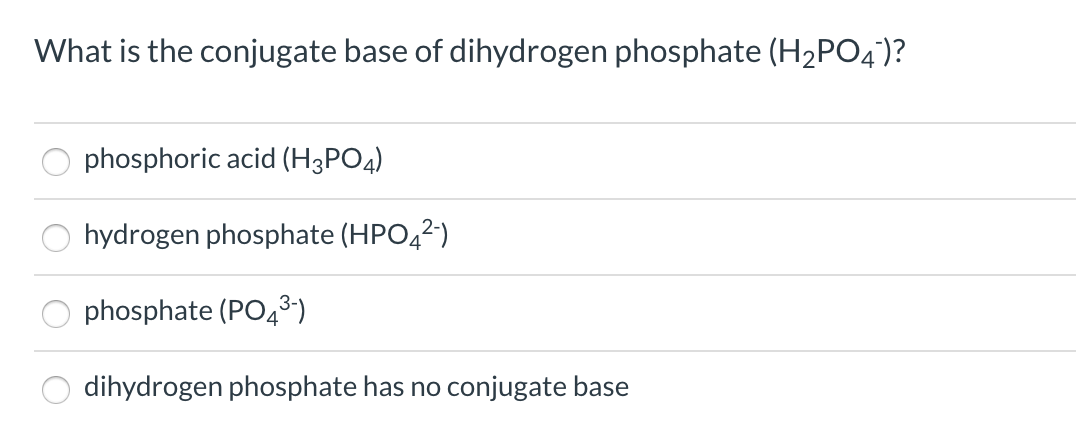
What is the conjugate base of H2PO4⁻? Which of the following is a WEAK acid? - Home Work Help - Learn CBSE Forum

⚗️HELP In the following acid-base reaction, NH4+ is the H2PO4- (aq) + NH3(aq) → HPO42- (aq) + - Brainly.com
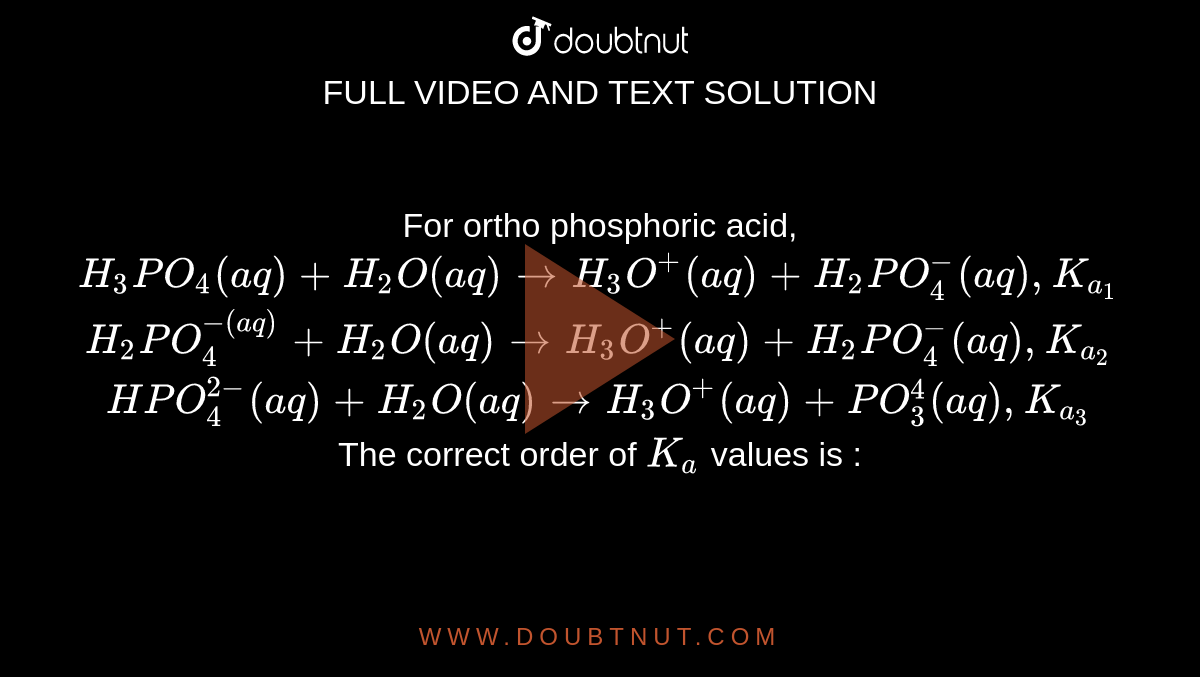


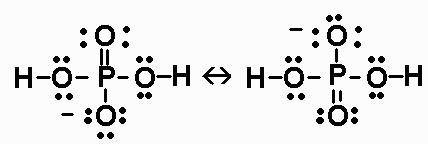


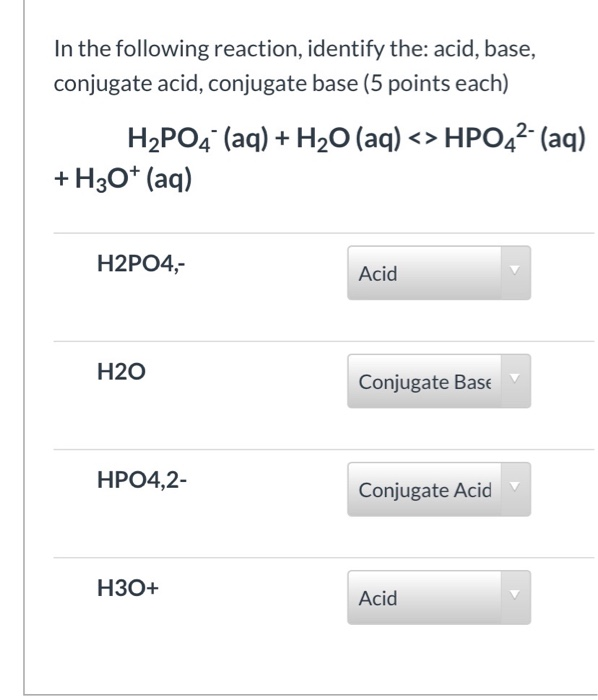




![ANSWERED] For the following equilibrium equation, c... - Physical Chemistry ANSWERED] For the following equilibrium equation, c... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/53256685-1659270432.1162956.jpeg)